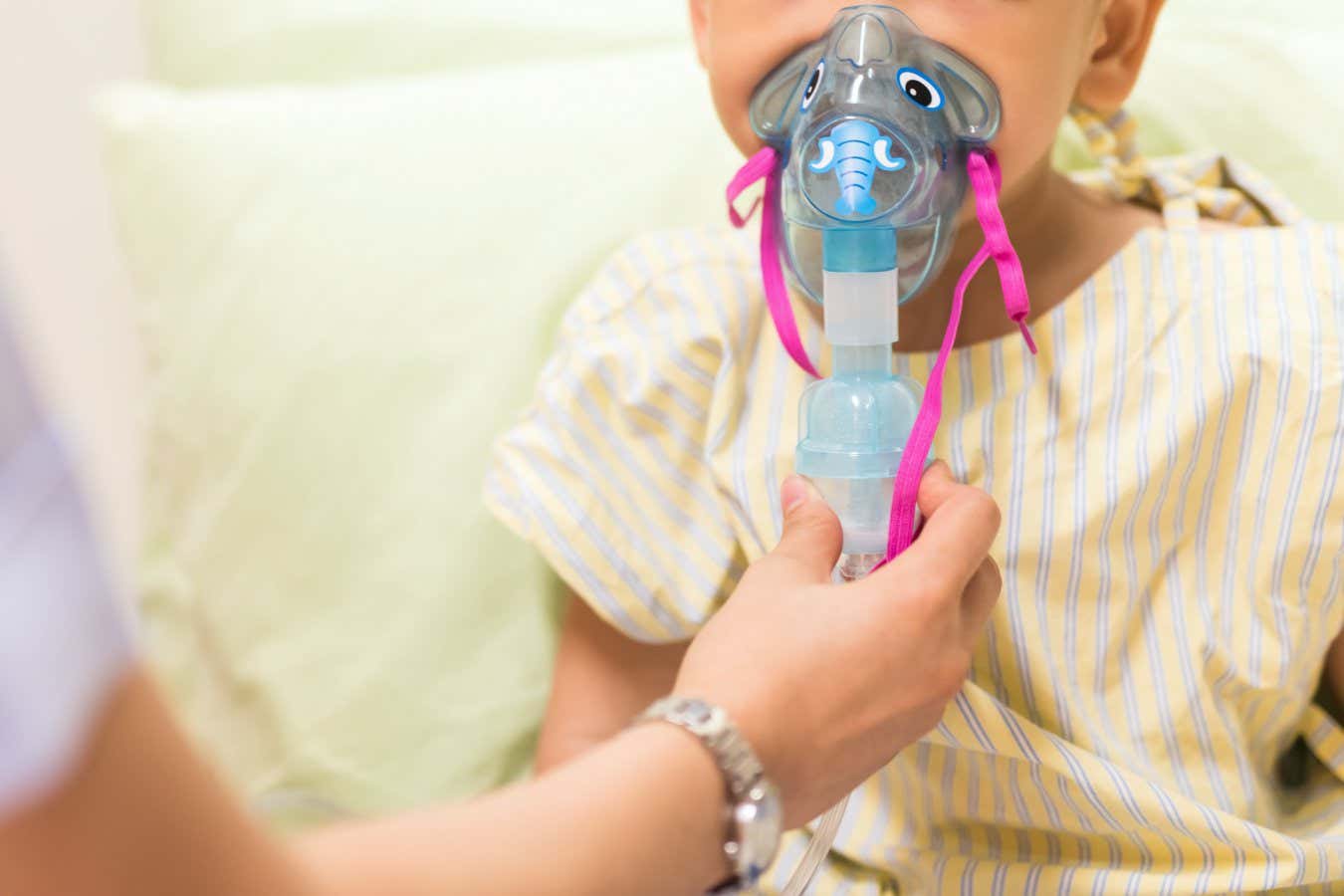
RSV can develop into respiratory diseases such as pneumonia
Shutterstock / Blanscape
The US Food and Drug Administration (FDA) has given approval to the first treatment for preventing severe respiratory syncytial virus (RSV) infections in children and infants.
RSV is a common virus that typically circulates during the autumn and winter seasons. While most children experience mild, cold-like symptoms with RSV, some, particularly those exposed to the virus for the first time, can develop life-threatening respiratory diseases including pneumonia and bronchiolitis.
The treatment, named Beyfortus, is administered as a single dose through injection before or during a baby’s first RSV season. Children up to 2 years old who continue to remain vulnerable to RSV during their second RSV season, like those with chronic lung or heart disease, are also eligible for the shot.
According to the FDA, approximately 1 to 3 percent of children under 1 year of age are hospitalized for RSV in the US each year. Last year’s RSV season was particularly severe, leading to medication shortages as hospitals across the country struggled to cope with the surge in cases.
“RSV can cause serious disease in infants and some children and results in a large number of emergency department and physician office visits each year,” said John Farley at the FDA’s Center for Drug Evaluation and Research in a statement. “Today’s approval addresses the great need for products to help reduce the impact of RSV disease on children, families, and the healthcare system.”
Beyfortus, produced by pharmaceutical company AstraZeneca, is not a vaccine. Instead, it utilizes monoclonal antibodies against RSV. These antibodies are engineered proteins that mimic the antibodies generated by the immune system to combat harmful pathogens. Unlike a vaccine, it does not train the body to recognize RSV or produce its own antibodies in response to infection. Common side effects include rash and pain, swelling, or redness at the injection site.
The FDA’s decision was based on data from three clinical trials involving nearly 4,000 infants. In these trials, a single dose of Beyfortus reduced the risk of infants needing medical care for an RSV infection by 70 to 75 percent.
Earlier this year, the FDA approved two vaccines against RSV for older adults. Later this year, the agency will also make a decision on a maternal RSV vaccine that would protect newborns from the illness.
Topics: